Diffusion MRI methods inspired by solid-state NMR
- Lund University, Physical Chemistry, Lund, Sweden
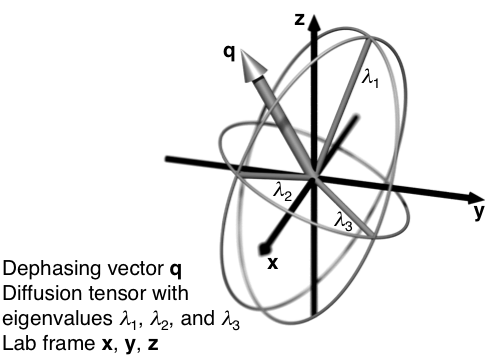
A wide range of porous materials, from lyotropic liquid crystals to brain tissue, contain anisotropic pores with varying sizes, shapes, and degrees of alignment on mesoscopic length scales. A complete characterization of the material requires estimation of all these parameters, but unfortunately their effects on the detected MRI signal are hopelessly entangled when using standard diffusion MRI methods. This presentation will give an overview of our recent work in adapting solid-state NMR methods to a diffusion MRI context, and thereby resolve the effects of pore size, anisotropy, and orientation. Our approach builds on the formal analogy between the chemical shift and diffusion anisotropy tensors, which means that classical solid-state NMR techniques, such as magic-angle spinning, have diffusion MRI equivalents [1]. As illustrated in the figure, in diffusion MRI the dephasing vector takes the role of the magnetic field vector in encoding the NMR signal with information about the size, shape, and orientation of the tensors. While solid-state NMR relies on physical reorientation of the sample in the magnetic field, diffusion MRI can utilize time-modulated magnetic field gradients to change the orientation of the dephasing vector with respect to the stationary sample. In its simplest form, magic-angle spinning of the dephasing vector allows for estimation of the distribution of isotropic diffusivities free from the confounding influence of anisotropy. Joint analysis of the directional and isotropic data yields quantitative estimates of both the shape [2] and the orientational order of the domains [3]. With numeric optimization of the gradient waveforms [4] the methods can be implemented on clinical MR scanners, for instance giving information about the shapes of randomly oriented cells in brain tumors [5].
[1] S. Eriksson, et al., J. Magn. Reson., 226, 13-18, (2013).
[2] S. Eriksson, et al., J. Chem. Phys., 142, 104201, (2015).
[3] S. Lasic, et al., Front. Physics, 2, 11, (2014).
[4] D. Topgaard, Microporous Mesoporous Mater., 178, 60-63, (2013).
[5] F. Szczepankiewicz, et al., Neuroimage, 104, 241-252, (2015).